Pharma production management is a critical aspect of the pharmaceutical industry that focuses on overseeing the efficient and effective production of drugs and medicines. It involves various activities, including planning work, organizing, and controlling the manufacturing processes to ensure the timely production of high-quality pharmaceutical products. This management function encompasses various areas such as resource allocation, scheduling, inventory management, quality assurance, and regulatory compliance.
By implementing rigorous production order management practices, pharmaceutical companies can optimize their manufacturing operations, minimize production costs, maintain strict quality standards, and ensure the availability of life-saving medications for patients worldwide. Effective pharma work order management is vital in meeting the growing demand for human well-being while adhering to stringent safety and regulatory requirements.
What is a work order in pharma manufacturing?
A production work order is a detailed document that guides the creation of a product at desired quantity within the provided timeline. It includes the product’s specifications, raw materials required, manufacturing steps, and quality control checks.
The production manager or team of experts familiar with the manufacturing process typically creates the production work order. It outlines the tasks that must be completed and the resources, personnel, and timelines required for each job. The process involves several steps, including any quality control checks if needed. The quality check ensures the highest quality standards of the products that meet all regulatory requirements.
Understanding of production work order
Pharmaceutical manufacturing is a highly regulated industry with complex processes that require close attention to detail. One critical aspect of pharma manufacturing is creating and managing production work orders. It defines the steps necessary to manufacture a product, including all the resources, personnel, and timelines needed.
Manufacturing pharmaceutical products is a complex process that requires careful planning and execution. The production work order is a critical tool that helps ensure that the manufacturing process runs smoothly and efficiently. It provides a roadmap for all the steps in creating a product, from the initial raw material procurement to the final packaging and distribution.
Implementing work order management solution
A software solution for work order management can significantly improve process efficiency and reliability. The following are some benefits of using software for work order management:
Benefits of using software for work order management
- Digitalization of production processes.
- Increased accuracy and consistency.
- Efficient resource allocation and personnel management.
- Faster production turnaround times.
- Improved communication and collaboration.
- Better tracking and monitoring of performance.

How to lead a pharmaceutical production work order?
Pharma manufacturing falls under the broad category of process manufacturing. In Process manufacturing, goods or products are created by combining supplies, raw materials, or ingredients using a pre-defined formula or recipe.
Drug manufacturing is the industrial-scale creation of pharmaceutical drugs by pharmaceutical companies. The means of drug manufacture can be broken down into a series of unit operations. Milling, granulation, coating, and Tablet pressing are all potential parts of the process.
The pharmaceutical industry has precise requirements and manufacturing guidelines. Therefore, pharmaceutical manufacturing equipment must comply with smart manufacturing practices. Pharmaceutical manufacturing equipment includes extensive equipment, such as capsule filling machines, x-ray inspection systems, tablet punches, and drying accessories. All processes can be automated to ensure precise manufacturing and formulation development.
The production order process in the pharma industry can vary depending on the specific manufacturing requirements and policies. Let us know how to create a work order in pharma manufacturing.
Planning:
The process begins with planning the production schedule and estimating the required resources, such as raw materials, personnel, and equipment.
Order Initiation:
A work order is generated once the production schedule is confirmed, indicating the product, quantity, and delivery date.
GMP (Good Manufacturing Practices) Compliance:
GMP in pharma is a set of regulations, guidelines, and procedures established by regulatory authorities to ensure that pharmaceutical products are consistently manufactured to a high quality and safety standard. The GMP goal is to be set before initiating production. GMP aims to ensure that every batch of a pharmaceutical product is safe, effective and meets the quality and purity standards required by regulatory agencies and consumers.
Material Procurement:
Raw materials required for manufacturing are procured from suppliers. Generally, suppliers are prequalified after verification of their GMP compliance.
Supply of Raw Materials:
The raw materials are measured and manually weighed or checked by computerized systems to ensure accuracy and traceability.
Granulation/Mixing:
In this step, the raw materials are mixed or granulated using specialized equipment per the formulation.
Compression
After granulation/mixing, the material is compressed to form tablets or capsules.
Coating:
In this step, the tablets are coated/painted with a uniform layer to improve the appearance, protect the contents, or control the release of drugs.
Quality Control:
Throughout the process, various quality checks and process controls are conducted at each stage to ensure the product’s safety, efficacy, and consistency.
Packaging:
Once the tablets/capsules pass quality control checks, they are packaged in bulk or prescribed quantities with appropriate labels.
Dispatch:
The finished product is dispatched to authorized sellers/ distributors, hospitals, or chemist shops for distribution or sale. The manufacturing work order process in the pharma industry involves careful planning, quality control and assurance, and stringent compliance with GMP and regulatory requirements.
How SFactrix MES helps Pharma Manufacturing?
SFactrix.ai MES provides the work order module to optimize manufacturing or production processes most efficiently. SFactrix.ai enables both discrete and process manufacturing industries to create and manage the process in real time. The complex pharma manufacturing process is no exception in streamlining their work order through SFactrix.
SFactrix is an IoT-enabled Smart Manufacturing Execution System that enables pharma manufacturing industries to digitalize production planning and execution and automate the entire factory floor operations. It is a one-stop solution for complete factory digitalization of the production process.
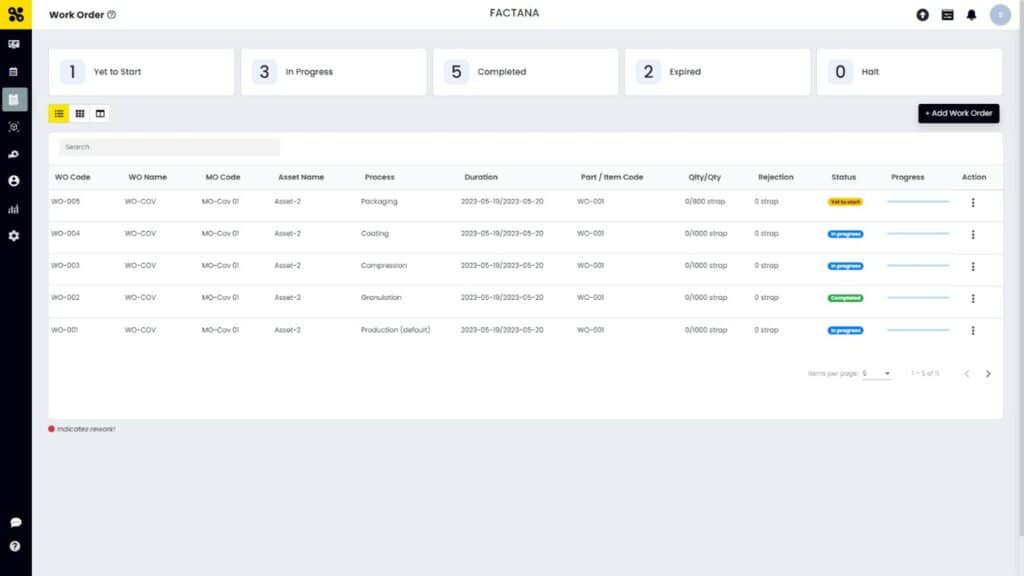
SFactrix can provide significant assistance to pharma manufacturers in work order management. Work order creation and management is a critical aspect of the manufacturing process in the pharmaceutical industry, involving the coordination and tracking of various tasks, resources, and timelines. SFactrix, as a comprehensive software solution, offers several key features and benefits that streamline and enhance work order management for pharma manufacturers.
Firstly, SFactrix enables creating and assigning work orders in a centralized platform. SFactrix eliminates manual paperwork and allows real-time tracking of production orders, ensuring improved visibility and transparency throughout the manufacturing process.
The software also facilitates effective resource management by providing insights into resource availability, allocation, and utilization. Work order management software helps pharma manufacturers optimize resource planning, efficiently allocating personnel and equipment to each work order.
Additionally, SFactrix allows for seamless collaboration and communication between departments involved in work order execution. It enables easy information sharing, updates, and documents, promoting better coordination and minimizing delays or errors.
Quality assurance or quality check is one of the crucial aspects of pharma manufacturing. SFactrix work order module offers quality monitoring on each work order process throughout the product manufacturing lifecycle. Each process in this module of SFactrix demonstrates the quality and rejection count.
Furthermore, SFactrix offers robust scheduling and tracking capabilities. It enables the creation of detailed timelines and milestones, ensuring work order execution within a pre-defined deadline.
SFactrix Smart MES solution is user-friendly, requiring no technical skills, no download or installation. It is cost-effective and requires no upfront investment.

Conclusion
As Pharma 4.0 is a growing trend in the global market, smart manufacturing or automated production is the need of the hour. Implementing a comprehensive and integrated platform for work order management will empower pharma manufacturing to deliver high-quality pharmaceutical products. To read more about pharma 4.0 in detail, read Pharma 4.0: The Future Of Pharmaceutical Manufacturing? (fogwing.io)

